Environmental view of teaching about heterogeneous catalysis:
An undergraduated laboratory experiment direct towards the remediation of water contamined with methylene blue
Regina de F P M Moreira*, Humberto J José,
Nivaldo C. Kuhnen, Angelina M. de L. Phillips,
Ticiane S. Pokrywiecki, Gercino Cesconetto Neto
Dept. of Chemical and Food Engineering, Univ. of Santa Catarina
Campus Universitário – Trindade
88040-970 Florianópolis – SC – Brazil
Phone: 55 048 3319448; Fax: 55 048 331 9687
e-mail: regina@enq.ufsc.brAbstract - This paper describes an undergraduate laboratory experiment on the kinetics of heterogeneous photocatalytic decolorization of methylene blue in aqueous solution. The experimental apparatus is simple and safe and allows the students to learn about Advanced Oxidation Processes, utilizing concepts of kinetics and heterogeneous catalysis.
Keywords: Advanced Oxidation Process; Photocatalysis; Kinetics; Undergraduate laboratory experiment
1. Introduction
Educational initiatives are important for the continued growth of Advanced Oxidation Processes. It is necessary to improve undergraduate science and engineering programs by incorporating more subject matter on wastewater treatment processes (1). Advanced Oxidation Processes are vital to many of the emerging engineering fields: scientists and engineers need to be knowledgeable in this area. The Federal University of Santa Catarina, Brazil has integrated Advanced Oxidation Processes into the chemical engineering curriculum and has developed model laboratory experiments directed towards learning about the latest technical advances and investigating methods of curriculum/laboratory development.
The effective teaching of photocatalysis is an important issue that needs to be addressed by the academic community. Educational initiatives are crucial to the continuation of technical growth and wide-scale commercialization involving Advanced Oxidation Processes. Laboratory experiments provide a unique opportunity to acquaint students with the principles and applications of photocatalysis, without significant curriculum modification.
This paper describes a safe and simple undergraduate laboratory experiment on the kinetics of heterogeneous photocatalytic decolorization of methylene blue in a slurry TiO2 reactor.
2. Background
Among the advanced oxidation processes, heterogeneous photocatalysis appears as an emerging destructive technology leading to the total mineralization of most of the organic pollutants.
When fine suspensions of TiO2 or other suitable semiconductors are irradiated at wavelengths less than 380 nm, this causes electron excitation from the valence band to the conduction band and a vacancy or hole is left in the valence bond. Such holes have the effect of a positive charge. This, in turn, generates the formation of “holes” on the surface of the semiconductor, which can react with oxygen, water, and hydroxide ion to form hydroxyl radical (2).
Furthermore, superoxide and perhydroxyl radicals are formed from the reaction of excited electrons with oxygen molecules. The highly reactive active oxygen species so formed then react with the organic pollutants resulting in their extensive oxidation. In many cases, carbon dioxide, water and inorganic ions are the final products.
The kinetics of photocatalytic degradation of many organic compounds in TiO2 dispersions under UV irradiation has often been modeled to the simple Langmuir-Hinshelwood equation (Eq. 1):
(1)
where r is the rate of disappearance of the reagent, C is the reagent concentration, k is the rate constant and K is the apparent equilibrium constant. The Langmuir-Hinshelwood equation is simplified to a pseudo first- order expression when the concentration of reagent is too low (Eq.2).
(2)
The integration of Equation 2 leads to Equation 3. The plot –ln(C/Co) versus t is a straight line and its slope is k’.
(3)
The photocatalytic photodegradation of methylene blue has been studied by several authors (3, 4) and the mechanistic scheme leading to the degradation of the dye is shown below.
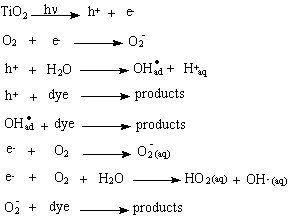
Carbon dioxide, nitrate, ammonium and sulfate ions are formed as products in the reaction, indicating the mineralization of the dye by illuminated TiO2 (4,5). The pH of the medium decreases and the conductivity increases during the reaction by the formation of these products.
3. Experiment Description
Materials: The photocatalyst employed was TiO2 (P-25, Degussa-Hülls). Methylene blue (Vetec) was used as received. NaOH or HCl 0,01M solutions were used to adjust the pH.
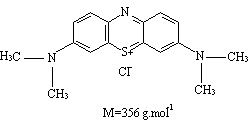
Methtylene blue structure
Experimental apparatus: The equipment required for this experiment was simple and safe. It consisted of a 2L glass reactor into which was placed a mechanical stirrer and a quartz vessel that separated the light source from the water. The light source was a medium pressure lamp (80W) without the cover glass.
The reactor was jacketed, and water was circulated outside it to avoid the heating of the solution.
Compressed air was continuously bubbled in the solution. The reactor provided two sample ports to accommodate the pHmeter and conductivimeter electrodes. The scheme of experimental apparatus is shown in Figure 1.
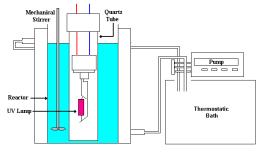
Figure 1 – Experimental apparatus
Procedure
The following is a summary of the procedure for this laboratory experiment as distributed to the students.
A volume of 2 L of the aqueous solution of methylene blue (Co in the range 27-100 mmol.L-1) is introduced into the reactor with 2.0 g of TiO2 (1 g.L-1) powder. The pH is adjusted to 9.0, using NaOH or HCl 0.01 mol.L-1.The suspension is stirred in the dark for 60 minutes before irradiation to achieve an equilibrated adsorption as deduced from the steady-state concentrations. Compressed air is continuously bubbled into the solution.
The pHmeter and conductivimeter electrodes are placed in the sample ports and then the UV lamp is turned on. Every 5 minutes, 5 mL of the solution is drained, and pH and conductivity is registered. The sample is filtered using PVDF membrane (0.22 m), and the absorbance of the solution is measured using a colorimeter or spectrophotometer set to a wavelength of 625 nm.
4. Results and discussion
The experimental results in this study are shown in Figure 2. Complete decolorization is reached in about 40 minutes of reaction.
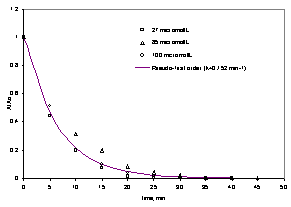
Figure 2 – Kinetics of decolorization of methylene blue mediated by TiO2/UV
Since the absorbance (A) is proportional to the concentration, according to Lambert-Beer Equation, the plot -ln(A/Ao) versus time is linear and the slope is directly k’ (Figure 3).
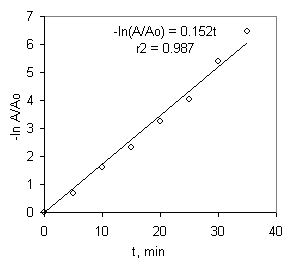
Figure 3 – Representation of a pseudo first- order kinetics (Co=100 mmol.L-1)
From the results shown in Figure 3 for the photocatalytic degradation at different initial concentrations, the students are enable to determine the value of the rate constant (k’). To heterogeneous photocatalytic degradation, k’ depends on the light source, amount of catalyst, pH and is independent on the initial concentration.
Least-square regression analysis of the data yields a value of the slope for this straight line (equal to k’) of 0.152 min-1. The students visualize the mineralization of methylene blue by the decolorization and by increasing the conductivity and decreasing the pH, as shown in Figure 4.
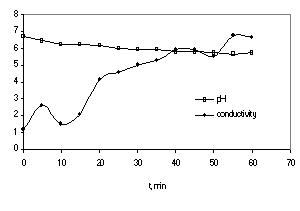
Figure 4 – Conductivity and pH of aqueous solution during the reaction
According to the stoichiometry of the overall oxidation reaction (Eq. 4), the pH of the reaction medium is expected to decrease.
(4)
However, pH in a UV-irradiated titania slurry is a rather complex parameter since it governs the water dissociation equilibrium, the surface charge of titania with respect to its point of zero charge and the ionization state of the organic reactant and of its metabolites (5). At pH higher than the point of zero charge of titania (pHpzc ~ 6,5 for TiO2), the surface becomes negatively charged and it is opposite for pH’s < pzc. Since methylene blue is a cationic dye, it is conceivable that at high pH’s its adsorption is favored on a negatively charged surface. Since the adsorption is a requirement for reaction, the reaction is faster at basic pH.
5. Conclusions
This paper reports a novel experiment for the determination of the kinetics of photocatalytic degradation of pollutants in aqueous solution. The experiment is safe and simple and is suitable for use in a variety of educational settings. It illustrates an experiment about the kinetics of heterogeneous catalytic reaction applied to wastewater treatment. This environmental view of heterogeneous catalysis is designed to develop a critical view about chemical processes and clean technologies. In addition to this, the experimental apparatus is also suitable to stimulate the student to toward further research activities.
6. References
1. Bumpus, J.A., Tricker, J., Andrzejewski, K., Rhoads, H., Tatarko, M., Remediation of water contaminated with azo dye: an undergraduate Laboratory experiment utilizing an inexpensive photocatalytic reactor, J. Chem. Educ., 76, 1680 (1998).
2. Hoffmann, M.R, Martin, S.T., Choi, W., Bahnemann, D., Environmental applications of semiconductor photocatalysis, Chemical Reviews, 95, 69-96 (1996).
3. Lakshmi, S., Renganathan, R., Fujita, S., Study on TiO2-mediated photocatalytic degradation of methylene blue, Journal of Photochemistry and Photobiology A: Chemistry, 88, 163-167 (1995).
4. Houas, A., Lachheb, H., Ksibi, M., Elaloiu, E., Guillard, C., Herrmann, J-M., Photocatalytic degradation pathway of methylene blue in water, Applied Catalysis B: Environmental, 31, 145-157 (2001).
5. Lachheb, H., Puzenat, E., Houas, A., Ksibi, M., Elaloui, E., Guillard, C., Herrmann, J.M., Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania, Applied Catalysis B: Environmental 39, 75-90 (2002).
Acknowledgments
UFSC – Federal University of Santa Catarina (Brazil) by financial support.